REC articles are not the view or opinion of Alpha Extract Administrators
Gold-DNA Nanosunflowers for Efficient Gene Silencing and Controlled Transformation
Mediame.guru
by Thamarasee Jeewandara , Phys.org
Developing an efficient delivery system for enhanced and controlled gene interference-based therapeutics is an existing challenge in molecular biology. The advancing field of nanotechnology can provide an effective, cross-disciplinary strategy to facilitate nucleic acid delivery. In a new report, Shuaidong Huo and colleagues in the interdisciplinary departments of Nanoscience, Interactive Materials, Chemistry and Polymer Research in China, Germany and the U.S. used triplex-forming oligonucleotide sequences coupled to its complementary strand to mediate the self-assembly of ultra-small gold nanoparticles.
The resulting sunflower-like nanostructures showed strong near infrared (NIR) absorption and ability for photothermal conversion. When the scientists irradiated the structures with NIR, the larger nanostructures disassembled to generate ultra-small nanoparticles modified with the c-Myc oncogene sequence to directly target the cancer cell nucleus. Huo et al. controlled gene silencing by synergistically controlling the time of preincubating cells with nanoparticles alongside nanostructure self-assembly (in vitro and in vivo) and the time-frame of NIR irradiation. The study provided a new paradigm to construct efficient and tailored nanocarriers for applications of gene interference and therapeutic gene delivery.
Gene therapy has great potential to treat a variety of diseases and complications including infertility, HIV and cancer. Successful gene therapy to alleviate disease symptoms depend on an efficient gene delivery vehicle or vector. During the process, the gene carrier must cross many biological barriers and cell membranes while escaping endosomal entrapment and nuclease-based degradation. Compared to virus-based delivery strategies, non-viral gene delivery approaches face many challenges during the process of loading and releasing DNA/RNA, targeted delivery and intracellular uptake, including incompatibility relative to immune responses in vivo.
Vigorous efforts in nanotechnology are underway to engineer stable and efficient vehicles for gene transfer to cancer cells. Due to their unique physiochemical properties a number of nanomaterials have emerged for gene delivery. Among them, gold nanoparticles (Au NPs) with specific size and surface properties can overcome obstacles in vivo to become one of the most studied gene carrier systems. However, these strategies have encountered a variety of shortcomings and therefore it is important to establish efficient delivery systems or enhanced and controlled gene therapies.
Self-assembly and testing sunflower-like nanostructures
In the present work, Huo et al. were inspired by nature's ability to hybridize DNA by engineering DNA-mediated, self-assembled gold DNA nanostructures (approximating 200 nm). The sunflower-like design showed strong NIR absorption and photothermal conversion properties. Upon NIR irradiation, the structures disassembled to liberate ultra-small gold nanoparticles (2 nm, Au NPs) with potential for oncogene silencing, improved cell and nuclei permeability and enhanced transfection efficiency. The scientists synergistically controlled the cell-nanomaterial interactions based on the time of pre-incubation in the lab, followed by time of circulation in vivo and the timeline of irradiation. The experiments facilitated increased cellular uptake, tunable gene silencing efficacy and controlled tumor inhibition. The transformable nanosunflowers provided an excellent model to design nanovehicles for drug delivery with great potential in biomedicine.
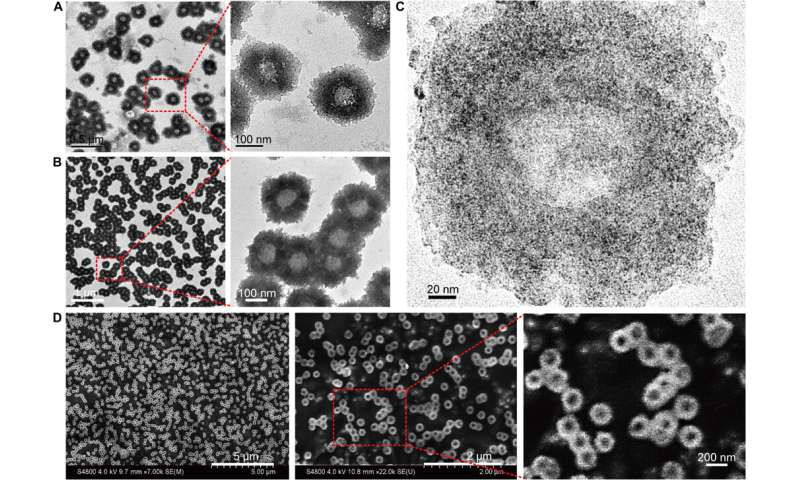
Huo et al. first synthesized the two-nanometer Au NPs coated with tiopronin and modified them with thiol-oligonucleotides (SH-POY2T) using an established method of ligand exchange. The 23-nucleotide (nt) POY2T oligonucleotide bound the P2 promoter of the c-myc oncogene to form a triplex structure and downregulate oncogenic c-myc expression. In parallel, they designed and synthesized another single-stranded sequence known as CA to complementarily hybridize to the tail of the POY2T sequence and block its binding to the c-myc oncogene. On completion, the nanostructure self-assembled into sunflower-like structures. The team investigated the nanostructure (200 nm) using transmission electron microscopy (TEM). Additional imaging revealed further details of the DNA moieties of the "sunflower" structure. When the materials scientists used scanning electron microscopy (SEM) to validate the TEM results, they observed consistency between the methods.
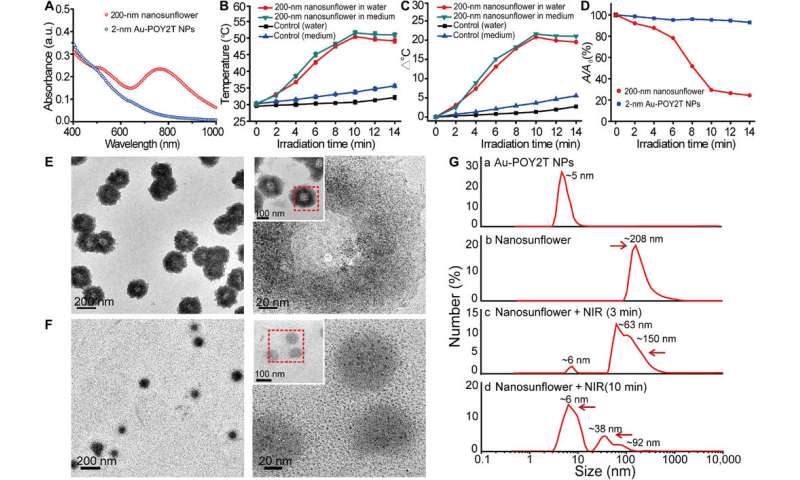
They investigated the UV-Vis absorption spectra of the ultrasmall Au NPs prior to DNA-mediated self-assembly. The monodispersed, individual two-nanometer Au-POY2T NPs showed strong absorption in the NIR region to generate heat under NIR irradiation. Huo et al. credited the observed strong NIR absorbance to close interparticle spacing and nonuniform spatial distribution of individual NPs within the larger nanostructure. They tested the heat response of the self-assembled nanostructures under NIR irradiation and noted the melting point of the complementary DNA sequences (POY2T and CA) to approximate 41 degrees C, dissociating half of the duplex structure between complementary DNA sequences. Huo et al. selected 10 minutes as the optimal time for NIR irradiation in the study.
Disassembly behaviour of the self-assembled nanostructures and proof-of-concept
The scientists hypothesized the self-assembled nanostructures would shrink and disassemble into individual ultrasmall Au-POY2T NPs. After 10 minutes of NIR irradiation, the maximum absorption (767 nm) of nanostructures markedly decreased to disassemble the sunflower structure. They followed the experiments before and after NIR irradiation with TEM observations and used particle size analysers to understand the disassembly process and size transformation of the nanostructures up to six nanometers in size and confirmed the optimal suitability of the 10-minute timeline.
Huo et al. applied NIR irradiation to MCF-7 cells treated with self-assembled gold DNA nanostructures and tested their cellular uptake in vitro as proof-of-concept. They determined the cellular internalization of Au-POY2T (2 nm) across diverse incubation times and quantified their cellular uptake using inductively coupled plasma mass spectroscopy (ICP-MS) and previous methods. They noted increased internalization after six hours of incubation compared to 24-hour incubation timelines. They did not observe inhibitors of endocytosis to influence Au-POY2T NP uptake, suggesting the involvement of an alternative path such as membrane fusion.
Understanding gene silencing behavior of the self-assembled nanostructures
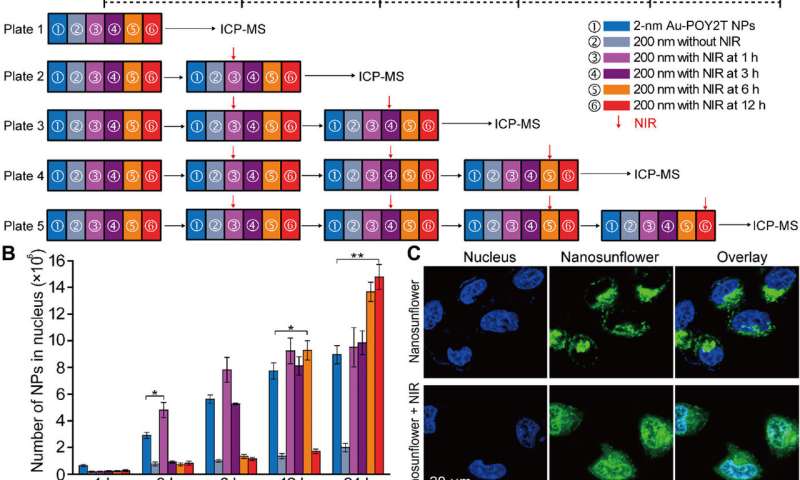
After enhanced cellular uptake of self-assembled nanostructures in vitro, the research team investigated the distribution of nanoparticles within the cell nuclei using "standby" and "attack" strategies after NIR triggering. For this, they extracted cell nuclei after incubation, for ICP-MS analysis after NIR irradiation across diverse periods of incubation (one, three, six and 12 hours). They noted that the pre-incubation period largely affects nanoparticle internalization within the cell nucleus, and the researchers regulated Au-POY2T NPs in the cell nucleus based on the time of pre-incubation and NIR irradiation.
Huo et al. also investigated NIR-irradiation controlled therapeutic effects of nanosunflowers using cell viability tests; they observed oncogene silencing to increase markedly (80 percent) and kill more cancer cells. The research team controlled the therapeutic impact effectively by changing the timeline of pre-incubation and irradiation efficiently. The results supported a superior ability of the transformable nanosunflowers to silence the c-myc oncogene and oncoprotein. The scientists controlled the gene silencing process by tuning pre-incubation timelines prior to NIR irradiation.
Controlling tumor growth inhibition using self-assembled nanosunflowers
To test the controllable anti-tumor efficiency of nanosunflowers in vivo, the scientists first investigated their blood compatibility to confirm good blood biocompatibility. The research team then established the MCF-7 tumor model using the BALB/c nude mice, allowed the tumor volumes to reach 50 mm3 and randomly divided the animals into nine groups and treated them with 1000 µl of varying POY2T formulations. After each injection, they irradiated the animal groups with NIR lasers for 10 minutes to reach a local temperature above 41 degrees C.
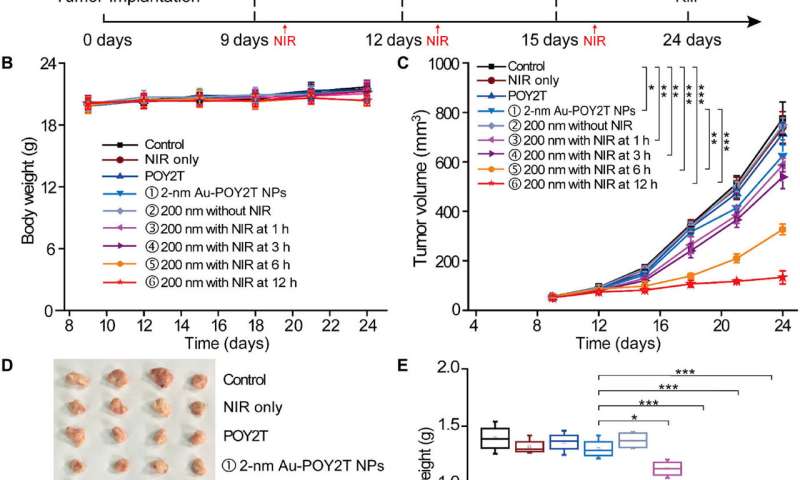
Of note, mice treated with the nanosunflower-treated group and irradiated at 12 hours showed the most significant anti-tumor effects, indicating efficient delivery of gene silencing units into the tumor site. After 24 days, Huo et al. sacrificed the animals, isolated the tumors and weighed them to demonstrate nanosunflower based NIR-controlled tumor growth inhibition in vivo. Based on histological studies, the team showed the treatment significantly reduced tumor growth and did not affect the morphology of other organs. The results verified the therapeutic efficiency and lack of side effects for nanosunflowers and NIR therapy.
In this way, Shuaidong Huo and colleagues designed, developed and optimized nanoagents for effective anti-tumor therapy. They engineered self-assembled sunflower-like nanostructures to act as multiparticle carriers loaded with many ultrasmall therapeutic units. Upon NIR irradiation, the nanostructures dissociated to release swarms of small NPs to target the cell nucleus. In tumor-bearing mice, the large sunflowers passively targeted the tumor site followed by NIR irradiation to transform the tumor genetic composition and shrink it. The research team aim to improve transfection efficiency and provide a blueprint for controllable gene silencing at tumor sites using transformable gene interference carriers for intricate theranostics at the level of the single cell.
Explore further
Reinhard Waehler et al. Engineering targeted viral vectors for gene therapy, Nature Reviews Genetics (2007). DOI: 10.1038/nrg2141
N. L. Rosi. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation, Science (2006). DOI: 10.1126/science.1125559
