Key Highlights
- A hemp-derived CBD nutraceutical for dogs developed by an Australian Animal Health Company, has substantially Improved Atopic Dermatitis symptoms in a canine clinical study;
- The product (DermaCann®) substantially reduced CADESI-4 scoring in dogs with Atopic Dermatitis, by an average of 51% after 56 days of treatment;
- CADESI-4 (Canine Atopic Dermatitis Extent and Severity Index) is a gold standard method used to grade inflammation and skin lesions in dogs with Atopic Dermatitis;
- DermaCann® was well tolerated by all dogs on treatment, with no significant adverse events reported throughout the length of the 8-week study;
- The Company is now in discussions with animal health partners to advance the commercialisation of DermaCann® in various regulatory and non-regulatory markets.
SYDNEY, July 21, 2020 /PRNewswire/ –Animal health company CannPal Animal Therapeutics Limited (ASX:CP1) (“CannPal” or “the Company”) has released results from its safety and efficacy study for DermaCann®, an oral nutraceutical developed for healthy skin and immune function for dogs.
Treatment with 2 similar DermaCann® formulations resulted in a substantial improvement in CADESI-4 scores, with an average reduction of 51% for dogs on treatment, compared to a slight increase observed in the placebo group between days 0 and 56.
CADESI-4 (Canine Atopic Dermatitis Extent and Severity Index) is a gold standard method used to grade skin lesions in clinical trials to assess the impact of treatments in dogs with Atopic Dermatitis.
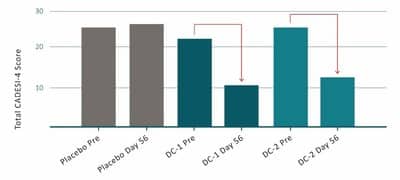
Fig 1: Mean of CADESI-4 scores in dogs treated with placebo or DermaCann® (DermaCann formulation 1 (DC-1) or DermaCann formulation 2 (DC-2)). Results from pre-treatment (day 0) to day 56. Lower score = treatment benefit.
Dosing for the safety and efficacy study commenced in Q4 2019 with 30 dogs expected to participate in the trial, however due to the social distancing measures implemented by the Australian Government in response to COVID-19, CannPal made the decision to finalise the study with 13 dogs having successfully completed treatment.
The study design was a randomised, double-blind, placebo-controlled clinical trial to assess the safety and efficacy of two similar DermaCann® formulations in dogs with dermatological skin conditions, using different sources of cannabidiol extracted from the hemp plant.
Dogs were dosed twice daily over a period of 56 days, with veterinary and owner assessments conducted on all dogs on (or around) Day 0, Day 28 and day 56.
Clinical assessments were completed by Dermatology Specialist Veterinarians using the validated CADESI-4 model to assess skin lesions in multiple areas classically affected by canine Atopic Dermatitis. Assessments of skin and coat health were also completed by the Dermatologists and dog owners.
There were no significant adverse events reported throughout and DermaCann® was well tolerated, with no dogs being withdrawn from the study.
CannPal will use the positive results from the trial as supportive efficacy data for the registration of DermaCann® in multiple markets as a nutraceutical for healthy skin and immune support for dogs.
The data has also been used to strengthen CannPals Intellectual Property portfolio, with the filing of the Company’s second PCT international (“PCT”) patent application. This application is expected to establish an exclusivity period for the Company’s proprietary DermaCann® formulation extending to mid-2040.
CannPal has also advanced discussions with various animal health partners to progress the commercialisation of DermaCann® in markets that may not require product registration due to the relaxing of regulations for hemp-derived CBD.

